Neurogenesis Promoted By Fluoxetine
Browsing on the Scientific American site, I came across a reference
to a new study: Fluoxetine targets early progenitor cells in
the adult brain. In was written by Juan M. Encinas,
Anne Vaahtokari, and Grigori Enikolopov; it was published online ahead
of print in PNAS
on May 15, 2006. The abstract is here;
the PDF (2.8MB), which is freely available, is here.
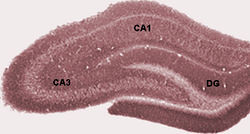 I
suppose that is true; we might someday develop a new treatment, based
upon this research. That is not guaranteed, though; even if
it does turn out to be the case, it would take decades to get a
marketable product out of it. Perhaps what is more
interesting is what is tells us about the mechanism of action of
antidepressant medication, and how the reality of what these drugs do
compares with popular misconceptions.
I
suppose that is true; we might someday develop a new treatment, based
upon this research. That is not guaranteed, though; even if
it does turn out to be the case, it would take decades to get a
marketable product out of it. Perhaps what is more
interesting is what is tells us about the mechanism of action of
antidepressant medication, and how the reality of what these drugs do
compares with popular misconceptions.
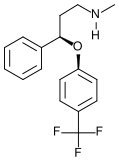 Fluoxetine
is the generic name for Prozac, the first selective serotonin reuptake
inhibitor marketed in the USA. Much has been made of the fact
that fluoxetine boosts the amount of serotonin in the neuronal synapse.
That effect is commonly thought of as the mechanism of action
of the drug. Skeptics of psychopharmacology are fond of
pointing out a problem with this hypothesis. The problem is
that the serotonin boost occurs within hours of taking the medication,
but the therapeutic effect takes weeks to develop. The
article by Encinas et. al. shows that the time
course for the development of new neurons takes about a month (in
mice). (On page 3 of the PDF file, there is an illustration
showing the progression from quiescent neural progenitor cells to
mature granule cells.)
Fluoxetine
is the generic name for Prozac, the first selective serotonin reuptake
inhibitor marketed in the USA. Much has been made of the fact
that fluoxetine boosts the amount of serotonin in the neuronal synapse.
That effect is commonly thought of as the mechanism of action
of the drug. Skeptics of psychopharmacology are fond of
pointing out a problem with this hypothesis. The problem is
that the serotonin boost occurs within hours of taking the medication,
but the therapeutic effect takes weeks to develop. The
article by Encinas et. al. shows that the time
course for the development of new neurons takes about a month (in
mice). (On page 3 of the PDF file, there is an illustration
showing the progression from quiescent neural progenitor cells to
mature granule cells.)
The thing is, one often starts to see clinical improvement about two weeks after the drug is started. That is well after the serotonin boost, but before any new neurons are fully developed. This makes it seem unlikely that neurogenesis is, by itself, responsible for the therapeutic effect. Of course, the validity of that line of reasoning is based upon the assumption that neurogenesis in humans is no faster than that in mice.
The demonstration of the existence of neurogenesis, and the time course, refutes the popular notion that the serotonin boost is the main mechanism of action. There is little doubt that the serotonin boost is an important part of how the drug works, but it clearly is not the whole story. The existence of neurogenesis also suggests that the notion, that antidepressants are basically "uppers" or a "quick fix," is misguided. Unlike the case with stimulants, people who take antidepressants do not notice anything immediately, except for side effects. Moreover, the action of an antidepressant drug takes place in three phases. In the first two weeks, we tend to see side effects, but little clinical effect. After two to eight weeks, there is reduction in symptoms. Over subsequent months, there is a reduction in the frequency of relapse. If the drug is stopped during the first several months of treatment, there is a high probability of relapse. That probability drops with each successive month, out for about nine to twelve months. It is possible that the relapse-reduction effect has a different neuronal basis than the immediate and intermediate effects.
It will be a while before we have a full understanding of all the effects of antidepressant medication. It will take even longer for us to map each of those neural effects to the clinical effects. I am tempted to speculate, at this point, that neurogenesis in not responsible for the clinical improvement that is seen in the first few weeks of treatment. It may, however, play a role in the reduced frequency of relapses.
Note: the images are public-domain, from Wikipedia. Click on them to go to the respective Wikipedia pages.
Fluoxetine targets early progenitor cells in the adult brainThis particular study is interesting to neuroscientists, mainly because it outlines a technique for studying what happens in the brain when certain medications are given. For nonspecialists, there are several points of interest. The Scientific American article points out one:
Juan M. Encinas, Anne Vaahtokari, and Grigori Enikolopov
Published online before print May 15, 2006
Proc. Natl. Acad. Sci. USA, 10.1073/pnas.0601992103
Chronic treatment with antidepressants increases neurogenesis in the adult hippocampus. This increase in the production of new neurons may be required for the behavioral effects of antidepressants. However, it is not known which class of cells within the neuronal differentiation cascade is targeted by the drugs. We have generated a reporter mouse line, which allows identification and classification of early neuronal progenitors. It also allows accurate quantitation of changes induced by neurogenic agents in these distinct subclasses of neuronal precursors. We use this line to demonstrate that the selective serotonin reuptake inhibitor antidepressant fluoxetine does not affect division of stem-like cells in the dentate gyrus but increases symmetric divisions of an early progenitor cell class. We further demonstrate that these cells are the sole class of neuronal progenitors targeted by fluoxetine in the adult brain and suggest that the fluoxetine-induced increase in new neurons arises as a result of the expansion of this cell class. This finding defines a cellular target for antidepressant drug therapies.
By isolating the step in neuron development that fluoxetine influences, the scientists have identified a new target for antidepressants that may have fewer side effects. The research also unveils the links in the chain leading from stem cells to new neurons as well as provides an animal tailor-made to investigate the mechanisms of other medicines and treatments, permitting a ray of hope into the darker regions of brain dysfunction.
 I
suppose that is true; we might someday develop a new treatment, based
upon this research. That is not guaranteed, though; even if
it does turn out to be the case, it would take decades to get a
marketable product out of it. Perhaps what is more
interesting is what is tells us about the mechanism of action of
antidepressant medication, and how the reality of what these drugs do
compares with popular misconceptions.
I
suppose that is true; we might someday develop a new treatment, based
upon this research. That is not guaranteed, though; even if
it does turn out to be the case, it would take decades to get a
marketable product out of it. Perhaps what is more
interesting is what is tells us about the mechanism of action of
antidepressant medication, and how the reality of what these drugs do
compares with popular misconceptions. Fluoxetine
is the generic name for Prozac, the first selective serotonin reuptake
inhibitor marketed in the USA. Much has been made of the fact
that fluoxetine boosts the amount of serotonin in the neuronal synapse.
That effect is commonly thought of as the mechanism of action
of the drug. Skeptics of psychopharmacology are fond of
pointing out a problem with this hypothesis. The problem is
that the serotonin boost occurs within hours of taking the medication,
but the therapeutic effect takes weeks to develop. The
article by Encinas et. al. shows that the time
course for the development of new neurons takes about a month (in
mice). (On page 3 of the PDF file, there is an illustration
showing the progression from quiescent neural progenitor cells to
mature granule cells.)
Fluoxetine
is the generic name for Prozac, the first selective serotonin reuptake
inhibitor marketed in the USA. Much has been made of the fact
that fluoxetine boosts the amount of serotonin in the neuronal synapse.
That effect is commonly thought of as the mechanism of action
of the drug. Skeptics of psychopharmacology are fond of
pointing out a problem with this hypothesis. The problem is
that the serotonin boost occurs within hours of taking the medication,
but the therapeutic effect takes weeks to develop. The
article by Encinas et. al. shows that the time
course for the development of new neurons takes about a month (in
mice). (On page 3 of the PDF file, there is an illustration
showing the progression from quiescent neural progenitor cells to
mature granule cells.) The thing is, one often starts to see clinical improvement about two weeks after the drug is started. That is well after the serotonin boost, but before any new neurons are fully developed. This makes it seem unlikely that neurogenesis is, by itself, responsible for the therapeutic effect. Of course, the validity of that line of reasoning is based upon the assumption that neurogenesis in humans is no faster than that in mice.
The demonstration of the existence of neurogenesis, and the time course, refutes the popular notion that the serotonin boost is the main mechanism of action. There is little doubt that the serotonin boost is an important part of how the drug works, but it clearly is not the whole story. The existence of neurogenesis also suggests that the notion, that antidepressants are basically "uppers" or a "quick fix," is misguided. Unlike the case with stimulants, people who take antidepressants do not notice anything immediately, except for side effects. Moreover, the action of an antidepressant drug takes place in three phases. In the first two weeks, we tend to see side effects, but little clinical effect. After two to eight weeks, there is reduction in symptoms. Over subsequent months, there is a reduction in the frequency of relapse. If the drug is stopped during the first several months of treatment, there is a high probability of relapse. That probability drops with each successive month, out for about nine to twelve months. It is possible that the relapse-reduction effect has a different neuronal basis than the immediate and intermediate effects.
It will be a while before we have a full understanding of all the effects of antidepressant medication. It will take even longer for us to map each of those neural effects to the clinical effects. I am tempted to speculate, at this point, that neurogenesis in not responsible for the clinical improvement that is seen in the first few weeks of treatment. It may, however, play a role in the reduced frequency of relapses.
Note: the images are public-domain, from Wikipedia. Click on them to go to the respective Wikipedia pages.

 :
Joseph j7uy5
:
Joseph j7uy5
<< Home